Thailand: Medical Device Regulations Change 2021
- ARQon

- Feb 2, 2021
- 2 min read
Updated: Feb 8, 2021
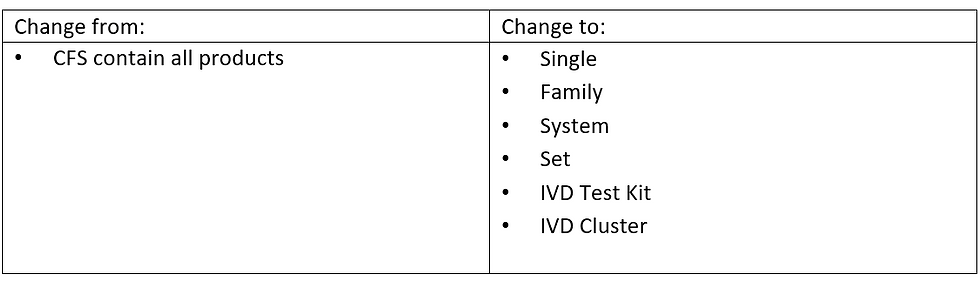
Product Classification:

Documents for Product Registration
• General class (CFS + ISO13485) change to Listing, Notified and Licensed
• Notification and Licensed class remain as notified and licensed
Difference between Existing Products and New Products

Each Submission
FDA Fee Changes

Registration – Components and Accessories
• Existing products - must list all accessories into FDA accessory system before transition
• Thai FDA will generate U code for each accessory for clearing customs
• Add into main System in new registration application
Missellaneous
• Existing license can still use until expired and renewal with new process condition
• New license is valid for 5 years
-> renewal as per classification
• Current CSDT submission submit each manufacturing site separately
->can grouping in one submission for various manufacturing sites
• FDA review fee is applied in first submission and exempted when renewal if no new information
Advertisment New Regulations
Advertisement direct to Healthcare Professionals
• Regulation – effective since Nov. 2, 2020
• No need to submit for pre-approval
• Only listing in Thai FDA system and can immediate implement
Exemption for Advertisement submission – only Trade name
• Gimmicks – effective since Nov. 5, 2020
• On back drop, poster, etc. – without any other claims of product features & benefit or motivation for demand
Label & IFU New Regulation (Effective on 31 Oct 2021)

Adverse Event & Field Safety Corrective Action Reporting
• AE occurred outside Thailand can be summarized to report twice a year only.
• AE in Jan-Jun – report within Aug
• AE in Jul-Dec – report within Feb next year
• The template of reporting has not yet announced, need to wait sub-regulation but must know before this August.
• For only event in Thailand need to report AE, malfunction and FSCA as current process.
• AE is only serious adverse event that leads to death, disable or need treatment or surgery to prevent permanent disable, public health hazard or near incident lead to AE.
• Effective Feb 5, 2021
Contact us at info@arqon.com
.png)



Comments